Electrochemistry: The Dance of Electrons and Chemical Change
Electrochemistry, a cornerstone of modern science and technology, is the branch of chemistry that explores the intricate relationship between electrical energy and chemical reactions. It delves into how chemical reactions can generate electricity and, conversely, how electrical energy can drive non-spontaneous chemical transformations. This bidirectional interplay forms the foundation for a vast array of technologies that permeate our daily lives, from the batteries powering our smartphones and electric vehicles to the industrial processes that produce essential materials and protect against corrosion.
The Foundation: Redox Reactions and Electrochemical Cells
At the heart of electrochemistry lies the concept of redox reactions, an abbreviation for reduction-oxidation reactions. These reactions are characterized by the transfer of electrons between chemical species, leading to changes in their oxidation states.
- Oxidation: This process involves the loss of electrons by a chemical species, resulting in an increase in its oxidation state. The species undergoing oxidation is referred to as the reducing agent because it causes another species to be reduced.
- Reduction: Conversely, reduction is the gain of electrons by a chemical species, leading to a decrease in its oxidation state. The species undergoing reduction is referred to as the oxidizing agent because it causes another species to be oxidized.
Crucially, oxidation and reduction are complementary processes that always occur simultaneously. One species cannot be oxidized without another species being reduced, and vice versa. This fundamental principle ensures the conservation of charge within the system.
Electrochemistry harnesses these redox reactions within specialized devices known as electrochemical cells. These cells are meticulously designed to either:
- Generate electricity from spontaneous redox reactions (Galvanic or Voltaic cells): These cells, commonly known as batteries, convert the chemical energy released during a spontaneous redox reaction into electrical energy that can be used to power external circuits.
- Use electrical energy to drive non-spontaneous redox reactions (Electrolytic cells): These cells utilize an external source of electrical energy to force a redox reaction to occur that would not otherwise proceed spontaneously. This principle is employed in various industrial processes, such as electroplating and the production of certain chemicals.
Delving Deeper: Types of Electrochemical Cells
Understanding the different types of electrochemical cells is crucial for appreciating the breadth and depth of electrochemistry.
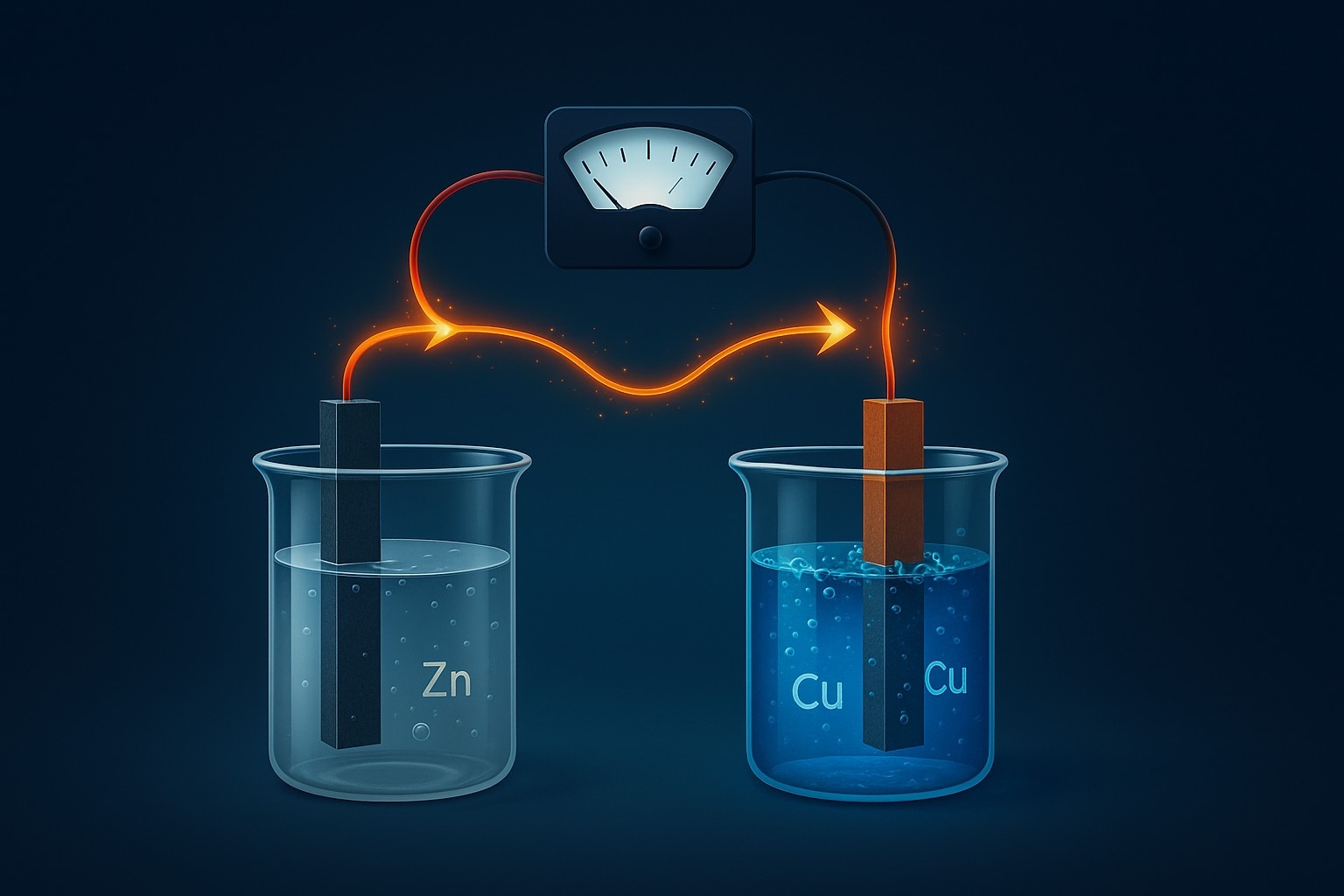
- Galvanic (Voltaic) Cells: These cells, named after Luigi Galvani and Alessandro Volta, are the workhorses of portable power. They convert chemical energy into electrical energy through spontaneous redox reactions. A typical galvanic cell consists of two half-cells, each containing an electrode immersed in an electrolyte solution. The electrodes are connected by an external circuit, allowing electrons to flow from the anode (where oxidation occurs) to the cathode (where reduction occurs). A crucial component is the salt bridge or porous membrane, which connects the two half-cells and maintains electrical neutrality by allowing the flow of ions. This prevents the buildup of charge in either half-cell, ensuring the continuous operation of the cell. A classic example is the Daniell cell, which utilizes zinc and copper electrodes immersed in solutions of their respective sulfates.
- Electrolytic Cells: These cells employ electrical energy to drive non-spontaneous redox reactions. Unlike galvanic cells, electrolytic cells require an external voltage source to force the reaction to occur. This external voltage provides the energy needed to overcome the thermodynamic barrier that prevents the reaction from proceeding spontaneously. The process involves applying a voltage across two electrodes immersed in an electrolyte solution. This forces electrons to flow in the opposite direction compared to a galvanic cell, causing oxidation at the anode and reduction at the cathode, even if the reaction would not occur naturally. A prime example is the electrolysis of water, where electrical energy is used to decompose water into hydrogen and oxygen gas. This process has significant implications for hydrogen production and energy storage.
Key Components and Terminology: A Language of Electrons
To navigate the world of electrochemistry, it’s essential to understand the key components and terminology:
- Electrodes: These are conductors where oxidation and reduction reactions take place. They serve as the interface between the electrolyte solution and the external circuit.
- Anode: The electrode where oxidation occurs. Electrons are released at the anode and flow into the external circuit.
- Cathode: The electrode where reduction occurs. Electrons are consumed at the cathode, facilitating the reduction of a chemical species.
- Electrolyte: This is a solution containing ions that conducts electricity. The electrolyte provides a medium for the movement of ions, which are essential for completing the circuit and maintaining charge balance within the electrochemical cell.
- Salt Bridge/Porous Membrane: This component connects the two half-cells in a galvanic cell, allowing the flow of ions to maintain electrical neutrality. The salt bridge typically contains an inert electrolyte, such as potassium chloride (KCl) or sodium nitrate (NaNO3), that does not interfere with the redox reactions occurring in the half-cells.
- Cell Potential (Voltage): This is the difference in electrical potential between the two electrodes, measured in volts (V). It represents the driving force of the redox reaction and indicates the amount of electrical energy that can be obtained from the cell.
- Standard Electrode Potential: This is the potential of a half-cell under standard conditions (298 K, 1 atm pressure, 1 M concentration) relative to the standard hydrogen electrode (SHE). The SHE is a reference electrode assigned a potential of 0 V, allowing for the comparison of the relative oxidizing and reducing strengths of different half-cells.
The Broad Spectrum of Applications: From Powering Devices to Protecting Materials
The principles of electrochemistry are applied in a vast array of fields, impacting numerous aspects of modern life:
- Batteries and Fuel Cells: These are the primary sources of portable power, powering everything from smartphones and laptops to electric vehicles and grid-scale energy storage systems. Batteries store chemical energy and convert it into electrical energy on demand, while fuel cells continuously convert chemical energy into electrical energy as long as fuel and oxidant are supplied.
- Corrosion Prevention: Understanding and mitigating the electrochemical processes that cause metal corrosion is crucial for extending the lifespan of infrastructure, vehicles, and other metallic structures. Techniques such as cathodic protection and the use of corrosion-resistant coatings are based on electrochemical principles.
- Electroplating: This process involves coating a metal surface with a thin layer of another metal for protection, aesthetic purposes, or to impart specific properties. Electroplating is widely used in the automotive, electronics, and jewelry industries.
- Electrolysis: This technique is used in the production of various chemicals, including chlorine gas, sodium hydroxide, and aluminum. Electrolysis is also used in the refining of metals and the production of hydrogen gas.
- Sensors: Electrochemical sensors are used to detect and quantify various substances in environmental monitoring, medical diagnostics, and industrial processes. These sensors rely on the electrochemical reactions that occur when the target substance interacts with the sensor’s electrode.
- Energy Storage: Developing advanced battery technologies and other energy storage solutions is crucial for transitioning to a sustainable energy future. Electrochemistry plays a central role in the design and development of new battery chemistries, supercapacitors, and other energy storage devices.
- Electrochemical Synthesis: This involves synthesizing organic and inorganic compounds using electrochemical methods. Electrochemical synthesis offers several advantages over traditional chemical synthesis, including milder reaction conditions, higher selectivity, and the potential for greener and more sustainable processes.
The Future of Electrochemistry : Innovation and Sustainability
Electrochemistry is a rapidly evolving field with ongoing research focused on addressing some of the most pressing challenges facing society:
- Developing more efficient and sustainable energy storage solutions: This includes research on new battery chemistries, such as lithium-sulfur, sodium-ion, and solid-state batteries, as well as the development of advanced supercapacitors and fuel cells.
- Improving corrosion resistance of materials: This involves developing new coatings and alloys that are less susceptible to corrosion, as well as exploring new methods for preventing corrosion in harsh environments.
- Developing new electrochemical sensors for a wide range of applications: This includes sensors for detecting pollutants in the environment, monitoring health conditions, and controlling industrial processes.
- Exploring new electrochemical methods for synthesizing chemicals: This includes developing more sustainable and efficient methods for producing pharmaceuticals, polymers, and other valuable chemicals.
- Electrocatalysis: This area focuses on developing new catalysts that can accelerate electrochemical reactions, leading to improved efficiency in energy conversion and storage devices.
Conclusion: A Field of Enduring Importance
Electrochemistry is a fundamental and versatile field that bridges the gap between chemistry and electricity. Its principles underpin a wide range of technologies that are essential to modern life. As we face challenges related to energy storage, environmental sustainability, and materials science, electrochemistry will continue to play a crucial role in developing innovative solutions for a better future. The dance of electrons and chemical change, orchestrated by the principles of electrochemistry, will continue to shape our world for generations to come.
– Dr. Pankaj Chauhan, M.Sc., Ph.D. (JRF)
Department of Chemistry, Madhav University