Sustainable Innovations for a Cleaner Future
Traditionally linked to heavy metals, aggressive conditions, and industrial processes, inorganic chemistry is quietly revolutionizing. As the world becomes increasingly environmentally conscious, chemists are embracing the principles of green chemistry to transform inorganic processes into cleaner, safer, and greener ones. Let’s see how environmentally friendly processes are revolutionizing the future of inorganic chemistry.
Principles of Green Chemistry in Inorganic Applications
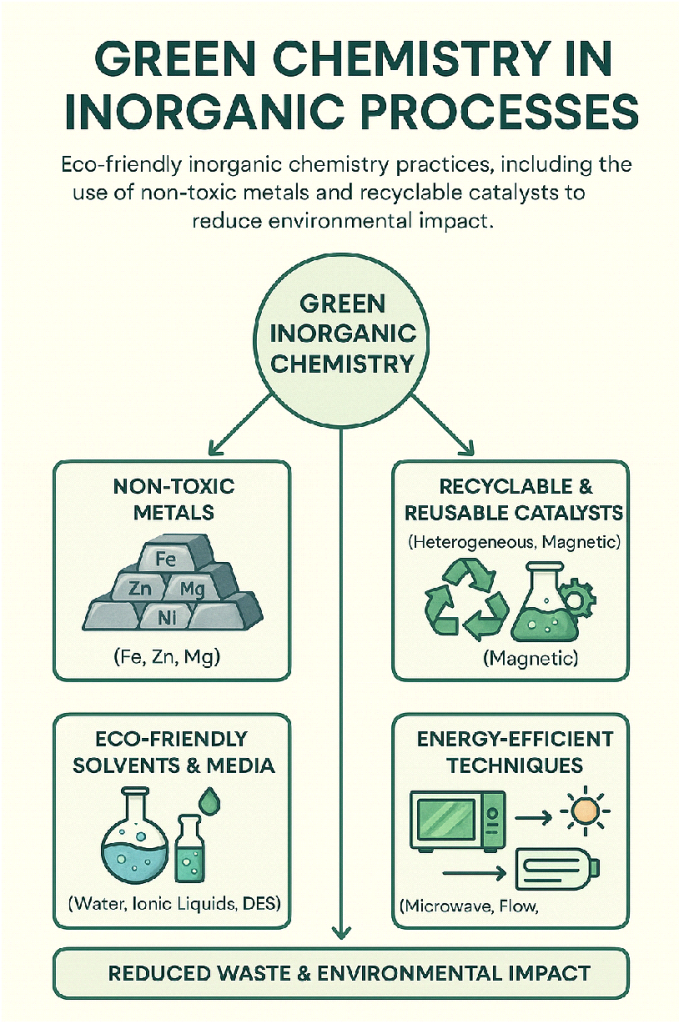
Green chemistry focuses on designing products and processes that reduce waste and have fewer hazardous materials. In inorganic chemistry, this means:
- Utilizing non-toxic, abundance metals rather than toxic or scarce transition metals.
- Utilizing recyclable and reusable catalysts with low metal leaching.
- Designing solvent-less or aqueous-phase reactions to remove dangerous organic solvents.
- Levying energy-efficient processes such as microwave-assisted synthesis and electrochemical methods.
Green Innovations in Inorganic Processes
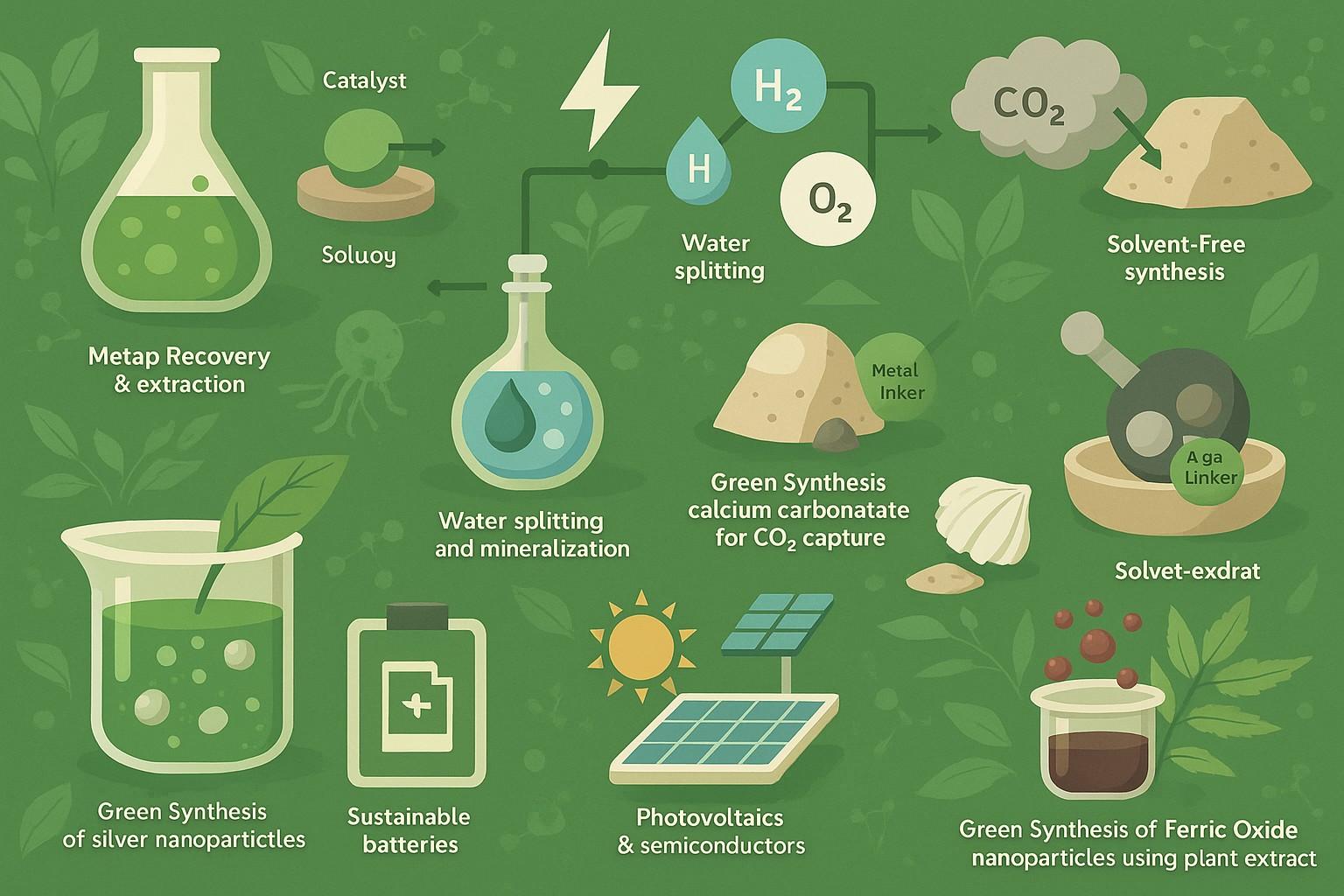
Metal Recovery and Extraction
Conventional metal extraction processes tend to depend on heat and aggressive chemicals. Bioleaching is one green hydrometallurgical process that employs microorganisms in extracting metals from ores at moderate conditions.
Ionic liquid-based extraction is another innovation, where volatile organic solvents are replaced with green solvents that can be recycled and adjusted for selectivity.
Catalysis and Green Catalysts
Catalysts are the backbone of most industrial inorganic processes. Scientists are presently developing heterogeneous catalysts (e.g., metal oxides on solid supports) that have low leaching and can be easily separated and reused.
Eg. Iron- and copper-based catalysts are substituting rare and toxic platinum group metals for oxidation and reduction reactions.
Magnetically recoverable nanoparticles provide a novel means for solvents-free separation of catalysts
Additionally, photocatalysts based on materials like titanium dioxide are enabling solar-powered chemical reactions.
Water Splitting and Hydrogen Production
Hydrogen is considered a green fuel-if it’s produced sustainably. Traditional methods use methane reforming, which emits CO₂. Green alternatives use electrocatalysts to drive water splitting using renewable energy.
Transition metal oxides (e.g., MnO₂, Co₃O₄) are showing promise as low-cost, earth-abundant materials for oxygen evolution reactions (OER).
CO₂ Utilization and Mineralization
Instead of looking at CO₂ as waste, inorganic chemistry is now looking to capture and convert CO₂ to stable carbonates through mineralization processes.
Calcium and magnesium silicates have been found to react with CO₂ to produce harmless solid carbonates, storing carbon forever and even creating materials that are beneficial for construction purposes.
Solvent-Free or Green Solvent Reactions
Reactions historically performed in organic solvents are being reengineered with supercritical CO₂, water, Ionic liquids or deep eutectic solvents, particularly in solid-state syntheses and crystallization processes.
Solvent free mechanochemical approaches, like ball milling, that do not use liquids at all.
Green Chemistry in Materials Development
Inorganic compounds have a central role to play in the development of energy, electronics, and transportation materials. Adopting green chemistry here translates to long-term environmental and economic advantages.
- Sustainable Batteries
Battery technology is dependent on inorganic materials such as lithium, cobalt, and nickel. Research aims at:
– Cobalt-free cathodes for lithium-ion batteries
– Solid-state electrolytes that do away with flammable liquid ingredients
– Efficient recycling technologies that reclaim metals with minimal environmental footprint
- Photovoltaics and Semiconductors
Production of semiconducting materials is usually done with toxic etchants and high temperatures. Green alternatives include:
– Solution-based fabrication of solar cells at room temperature
– Utilization of lead-free perovskites for next-generation photovoltaics
Case Studies
Green Synthesis of Silver Nanoparticles Using Tea Extract
Silver nanoparticles are made quickly and sustainably with Camellia sinensis (green tea) extract. This extract acts as a reducing and stabilizing agent. This method avoids harmful chemicals and produces nanoparticles with strong antimicrobial properties. They are suitable for wound healing, water purification, and antibacterial coatings.
Photocatalytic Water Splitting with Iron-Doped TiO₂
Iron-doped titanium dioxide (TiO₂) improves visible light absorption. This allows for efficient hydrogen production through sunlight-driven water splitting. This green method provides a low-energy option for clean hydrogen fuel generation.
Waste Shell-Derived Calcium Carbonate for CO₂ Capture
Calcium carbonate from bio-waste like oyster or egg shells works as an effective CO₂ absorber. This process reuses waste material to cut carbon emissions. It is a cost-effective and sustainable solution for treating industrial flue gas.
Solvent-Free Synthesis of Metal-Organic Frameworks (MOFs)
MOFs can be made without toxic solvents by grinding metal salts with organic linkers at room temperature. This solvent-free method saves energy and produces high-surface-area materials that are perfect for gas storage, catalysis, and drug delivery.
Green Synthesis of Ferric Oxide Nanoparticles Using Plant Extracts
Ferric oxide (Fe₂O₃) nanoparticles are created using neem (Azadirachta indica) leaf extract, which reduces Fe³⁺ ions under gentle conditions. This green method avoids harmful reagents, saves energy, and produces biocompatible nanoparticles that are useful in catalysis, environmental cleanup, and targeted drug delivery.
Green inorganic chemistry is not only about replacing toxic substances—it’s about redesigning processes at the molecular level to promote sustainability, efficiency, and circularity
Conclusion: A Cleaner Future with Inorganic Innovation
The application of green chemistry principles in inorganic processes is no longer a conceptual ideal—it’s a practical reality shaping modern science and industry. Through the use of non-toxic materials, recyclable catalysts, green solvents, and energy-saving methods, inorganic chemists are redefining what’s possible in a field long dominated by efficiency at the expense of sustainability.
– Dr. Afsar Ali, Associate Professor
Department of Chemistry, FOBAS, Madhav University